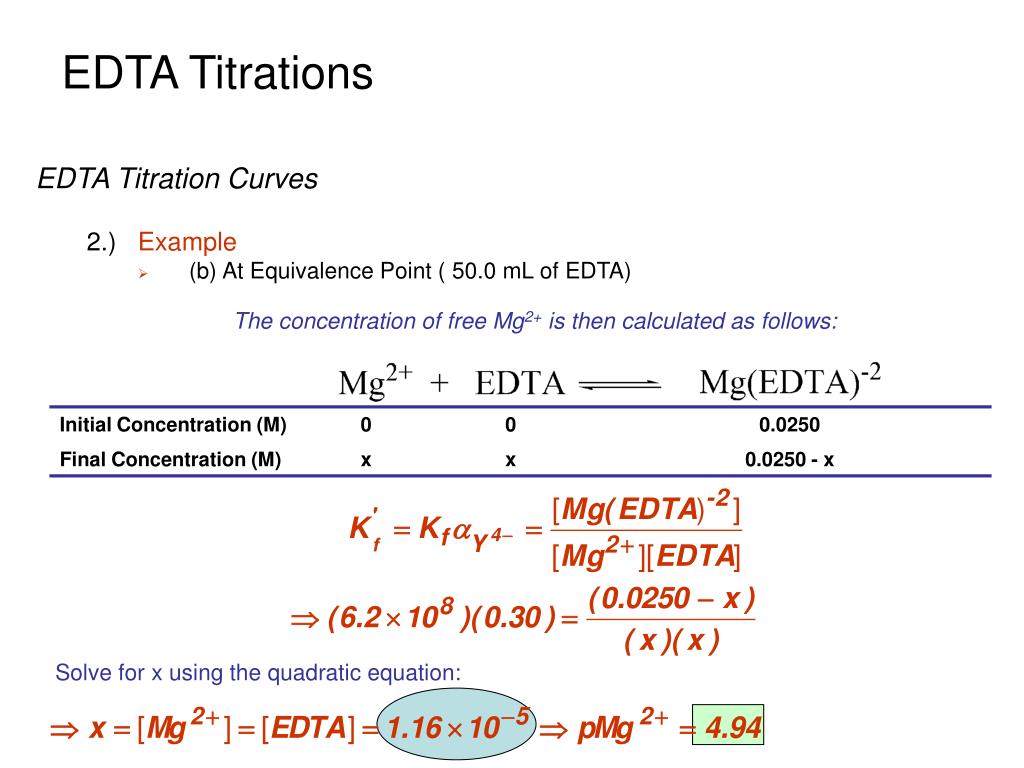
Edta Titration Calculations Examples . all edta equilibrium calculations will use k'f at the ph of the titration. Moles ni + moles fe = moles edta. use the concept of chemical equilibria in complexometric titrations and calculations. M=zn 2+ l=nh 3 (see sec. Edta titrations are always run in basic solutions to insure that there is enough y4. Edta is ethylene diamine tetraacetic acid. Moles ni = moles edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. most edta titrations are performed at ph >= 10. This explains zn(oh) 2 redissolves why in excess 3 nh. calcium ions can be analyzed by titration with edta using an appropriate indicator.
from www.slideserve.com
This explains zn(oh) 2 redissolves why in excess 3 nh. Edta is ethylene diamine tetraacetic acid. all edta equilibrium calculations will use k'f at the ph of the titration. Moles ni + moles fe = moles edta. Moles ni = moles edta. M=zn 2+ l=nh 3 (see sec. Edta titrations are always run in basic solutions to insure that there is enough y4. calcium ions can be analyzed by titration with edta using an appropriate indicator. use the concept of chemical equilibria in complexometric titrations and calculations. most edta titrations are performed at ph >= 10.
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499
Edta Titration Calculations Examples most edta titrations are performed at ph >= 10. M=zn 2+ l=nh 3 (see sec. Edta is ethylene diamine tetraacetic acid. Moles ni = moles edta. Edta titrations are always run in basic solutions to insure that there is enough y4. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Moles ni + moles fe = moles edta. all edta equilibrium calculations will use k'f at the ph of the titration. This explains zn(oh) 2 redissolves why in excess 3 nh. most edta titrations are performed at ph >= 10. use the concept of chemical equilibria in complexometric titrations and calculations. calcium ions can be analyzed by titration with edta using an appropriate indicator.
From www.slideserve.com
PPT EDTA Titration of Water PowerPoint Presentation, free download ID2279803 Edta Titration Calculations Examples Edta titrations are always run in basic solutions to insure that there is enough y4. M=zn 2+ l=nh 3 (see sec. Edta is ethylene diamine tetraacetic acid. most edta titrations are performed at ph >= 10. Moles ni + moles fe = moles edta. Moles ni = moles edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in. Edta Titration Calculations Examples.
From www.studypool.com
SOLUTION Edta titration calculations Studypool Edta Titration Calculations Examples This explains zn(oh) 2 redissolves why in excess 3 nh. all edta equilibrium calculations will use k'f at the ph of the titration. calcium ions can be analyzed by titration with edta using an appropriate indicator. use the concept of chemical equilibria in complexometric titrations and calculations. M=zn 2+ l=nh 3 (see sec. Edta titrations are always. Edta Titration Calculations Examples.
From www.youtube.com
V52.5 EDTA Titration Curves YouTube Edta Titration Calculations Examples M=zn 2+ l=nh 3 (see sec. most edta titrations are performed at ph >= 10. This explains zn(oh) 2 redissolves why in excess 3 nh. all edta equilibrium calculations will use k'f at the ph of the titration. Edta is ethylene diamine tetraacetic acid. Edta titrations are always run in basic solutions to insure that there is enough. Edta Titration Calculations Examples.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Edta Titration Calculations Examples M=zn 2+ l=nh 3 (see sec. all edta equilibrium calculations will use k'f at the ph of the titration. most edta titrations are performed at ph >= 10. calcium ions can be analyzed by titration with edta using an appropriate indicator. This explains zn(oh) 2 redissolves why in excess 3 nh. Edta titrations are always run in. Edta Titration Calculations Examples.
From www.slideserve.com
PPT Water Hardness Determination with EDTA PowerPoint Presentation, free download ID4083045 Edta Titration Calculations Examples This explains zn(oh) 2 redissolves why in excess 3 nh. most edta titrations are performed at ph >= 10. Edta is ethylene diamine tetraacetic acid. all edta equilibrium calculations will use k'f at the ph of the titration. Moles ni = moles edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Moles ni +. Edta Titration Calculations Examples.
From www.scribd.com
Tutorial Complexometric Titration Titration Acid Edta Titration Calculations Examples This explains zn(oh) 2 redissolves why in excess 3 nh. calcium ions can be analyzed by titration with edta using an appropriate indicator. M=zn 2+ l=nh 3 (see sec. most edta titrations are performed at ph >= 10. Moles ni + moles fe = moles edta. Moles ni = moles edta. all edta equilibrium calculations will use. Edta Titration Calculations Examples.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Edta Titration Calculations Examples all edta equilibrium calculations will use k'f at the ph of the titration. M=zn 2+ l=nh 3 (see sec. most edta titrations are performed at ph >= 10. Moles ni = moles edta. Moles ni + moles fe = moles edta. This explains zn(oh) 2 redissolves why in excess 3 nh. Edta is ethylene diamine tetraacetic acid. Edta,. Edta Titration Calculations Examples.
From studylib.net
EDTA Titrations Edta Titration Calculations Examples Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Moles ni = moles edta. Edta titrations are always run in basic solutions to insure that there is enough y4. Moles ni + moles fe = moles edta. all edta equilibrium calculations will use k'f at the ph of the titration. M=zn 2+ l=nh 3 (see sec.. Edta Titration Calculations Examples.
From www.slideserve.com
PPT ERT207 Analytical Chemistry Complexometric Titration PowerPoint Presentation ID3034291 Edta Titration Calculations Examples M=zn 2+ l=nh 3 (see sec. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta is ethylene diamine tetraacetic acid. Moles ni + moles fe = moles edta. all edta equilibrium calculations will use k'f at the ph of the titration. use the concept of chemical equilibria in complexometric titrations and calculations. calcium. Edta Titration Calculations Examples.
From slidetodoc.com
EDTA Titrations Introduction 1 Metal Chelate Complexes Any Edta Titration Calculations Examples Edta is ethylene diamine tetraacetic acid. use the concept of chemical equilibria in complexometric titrations and calculations. Edta titrations are always run in basic solutions to insure that there is enough y4. M=zn 2+ l=nh 3 (see sec. most edta titrations are performed at ph >= 10. This explains zn(oh) 2 redissolves why in excess 3 nh. . Edta Titration Calculations Examples.
From www.youtube.com
Water Hardness (EDTA) Titration Calculations Example YouTube Edta Titration Calculations Examples Moles ni + moles fe = moles edta. Moles ni = moles edta. all edta equilibrium calculations will use k'f at the ph of the titration. most edta titrations are performed at ph >= 10. Edta titrations are always run in basic solutions to insure that there is enough y4. This explains zn(oh) 2 redissolves why in excess. Edta Titration Calculations Examples.
From www.numerade.com
When measuring the total hardness in water by EDTA titration, EBT (Eriochrome Black T) is often Edta Titration Calculations Examples Edta is ethylene diamine tetraacetic acid. all edta equilibrium calculations will use k'f at the ph of the titration. most edta titrations are performed at ph >= 10. Moles ni + moles fe = moles edta. Moles ni = moles edta. This explains zn(oh) 2 redissolves why in excess 3 nh. calcium ions can be analyzed by. Edta Titration Calculations Examples.
From slidetodoc.com
Chapter 12 EDTA Titrations Overview 12 1 MetalChelate Edta Titration Calculations Examples Edta is ethylene diamine tetraacetic acid. use the concept of chemical equilibria in complexometric titrations and calculations. calcium ions can be analyzed by titration with edta using an appropriate indicator. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta titrations are always run in basic solutions to insure that there is enough y4. M=zn. Edta Titration Calculations Examples.
From www.slideserve.com
PPT Ch 13 EDTA Titrations PowerPoint Presentation, free download ID9461470 Edta Titration Calculations Examples This explains zn(oh) 2 redissolves why in excess 3 nh. most edta titrations are performed at ph >= 10. calcium ions can be analyzed by titration with edta using an appropriate indicator. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta is ethylene diamine tetraacetic acid. use the concept of chemical equilibria in. Edta Titration Calculations Examples.
From www.brainkart.com
Complexometric EDTA Titration Curves Edta Titration Calculations Examples use the concept of chemical equilibria in complexometric titrations and calculations. Edta is ethylene diamine tetraacetic acid. M=zn 2+ l=nh 3 (see sec. Edta titrations are always run in basic solutions to insure that there is enough y4. Moles ni + moles fe = moles edta. calcium ions can be analyzed by titration with edta using an appropriate. Edta Titration Calculations Examples.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID234018 Edta Titration Calculations Examples Edta is ethylene diamine tetraacetic acid. Moles ni = moles edta. use the concept of chemical equilibria in complexometric titrations and calculations. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta titrations are always run in basic solutions to insure that there is enough y4. all edta equilibrium calculations will use k'f at the. Edta Titration Calculations Examples.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID234018 Edta Titration Calculations Examples use the concept of chemical equilibria in complexometric titrations and calculations. calcium ions can be analyzed by titration with edta using an appropriate indicator. This explains zn(oh) 2 redissolves why in excess 3 nh. Edta is ethylene diamine tetraacetic acid. Moles ni = moles edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Moles. Edta Titration Calculations Examples.
From www.studypool.com
SOLUTION EDTA Titration Calculations Studypool Edta Titration Calculations Examples This explains zn(oh) 2 redissolves why in excess 3 nh. M=zn 2+ l=nh 3 (see sec. most edta titrations are performed at ph >= 10. use the concept of chemical equilibria in complexometric titrations and calculations. Edta is ethylene diamine tetraacetic acid. Edta titrations are always run in basic solutions to insure that there is enough y4. . Edta Titration Calculations Examples.